White Paper
How Top Sponsors Build
Strong Site Relationships That Last
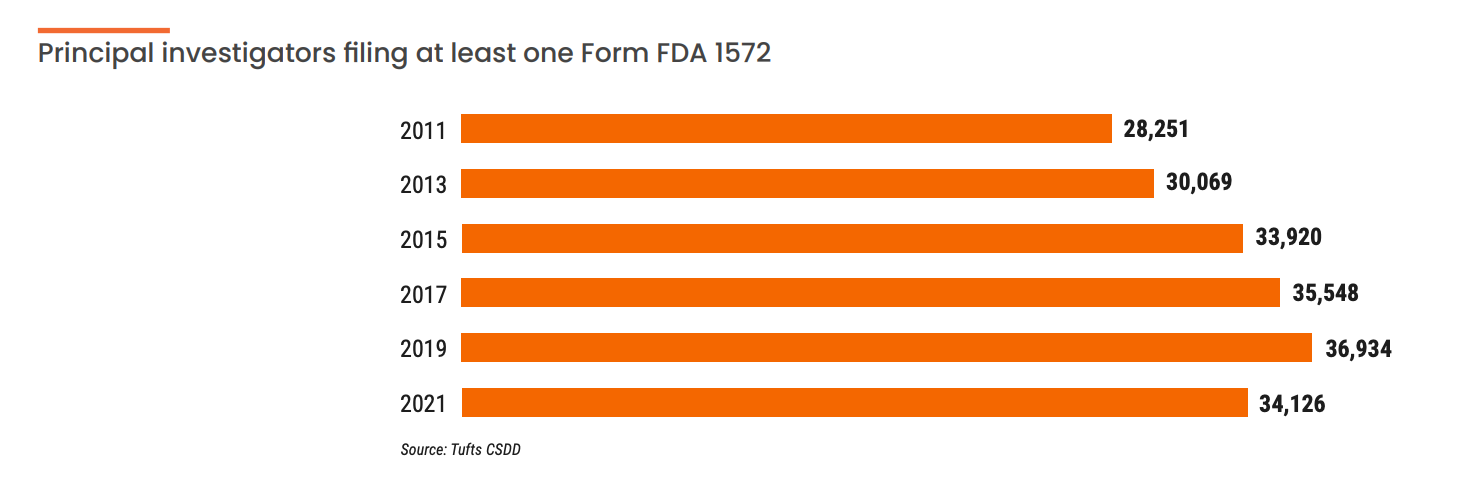
In the past five years, there’s been an explosion of new sponsor and vendor technology made to improve clinical research site engagement. But, these wellintentioned solutions are often custom, standalone systems that require complex middleware and integrations. This forces sites to adopt dozens of sponsor-specific tools and build customized processes around them. This is just one of the reasons an estimated 3,000 clinical research sites have stopped conducting trials since 2019, according to Ken Getz, executive director of the Tufts Center for the Study of Drug Development (CSDD).
Although most sponsors understand the urgency and value of improving sponsorsite relationships, it can be challenging to implement effective strategies that don’t exacerbate existing technology siloes. New data from Tufts CSDD captures the current environment for global sites. Natalie Blake, director in the global clinical trials organization at Merck & Co., Inc., Rahway, NJ, USA (hereinafter "MSD"), and Karen Whitson, associate director of study operations at AbbVie, share how they’ve improved site collaboration despite these industry challenges.
“The exodus of lower volume and less experienced sites with modest infrastructure has caused larger sites, health systems, and academic medical centers to pick up more activity.” - Ken Getz, Executive Director of the Tufts Center for the Study of Drug Development
Unpacking current conditions for clinical research sites
The majority of the 3,000 sites that have stopped conducting trials were small, community-based independent centers conducting one or two clinical trials annually. “This exodus of lower volume and less experienced sites with modest infrastructure has caused larger sites, health systems, and academic medical centers to pick up more activity,” says Getz.
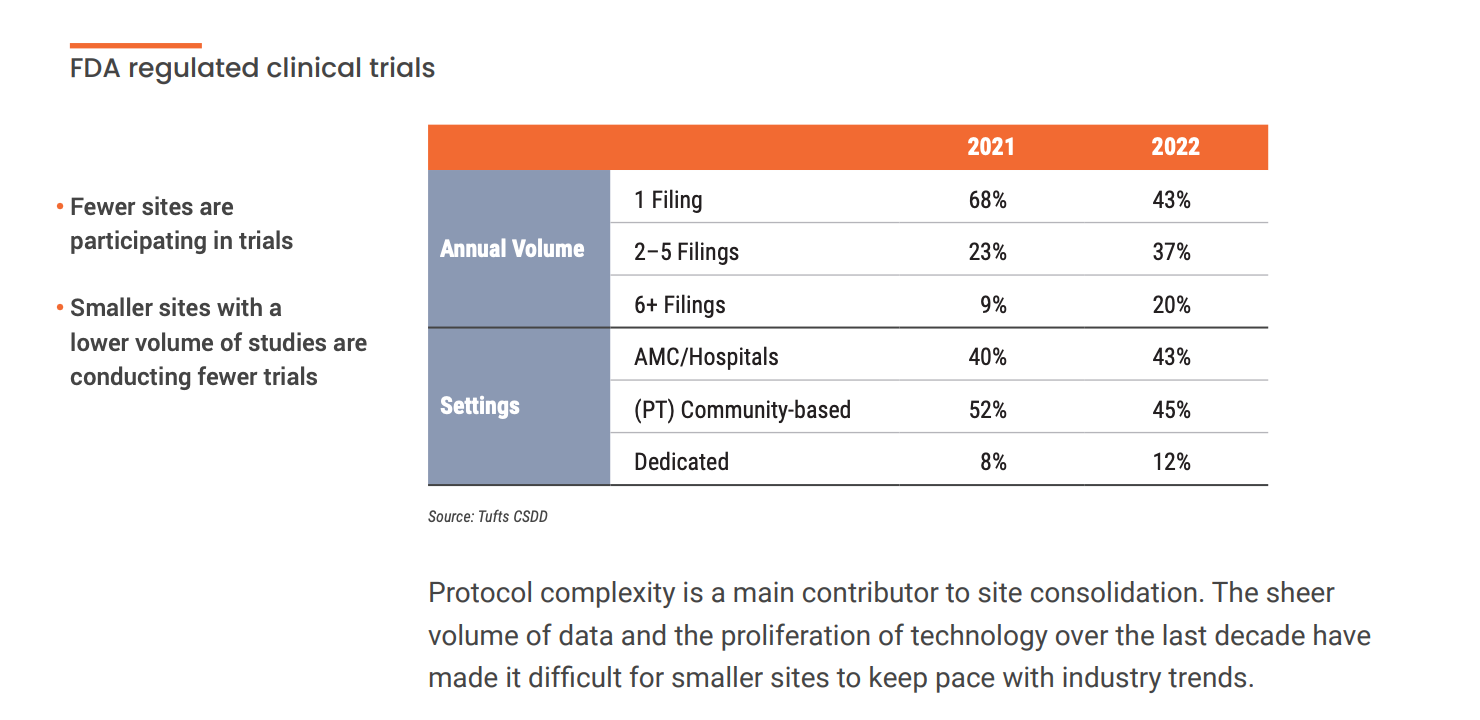
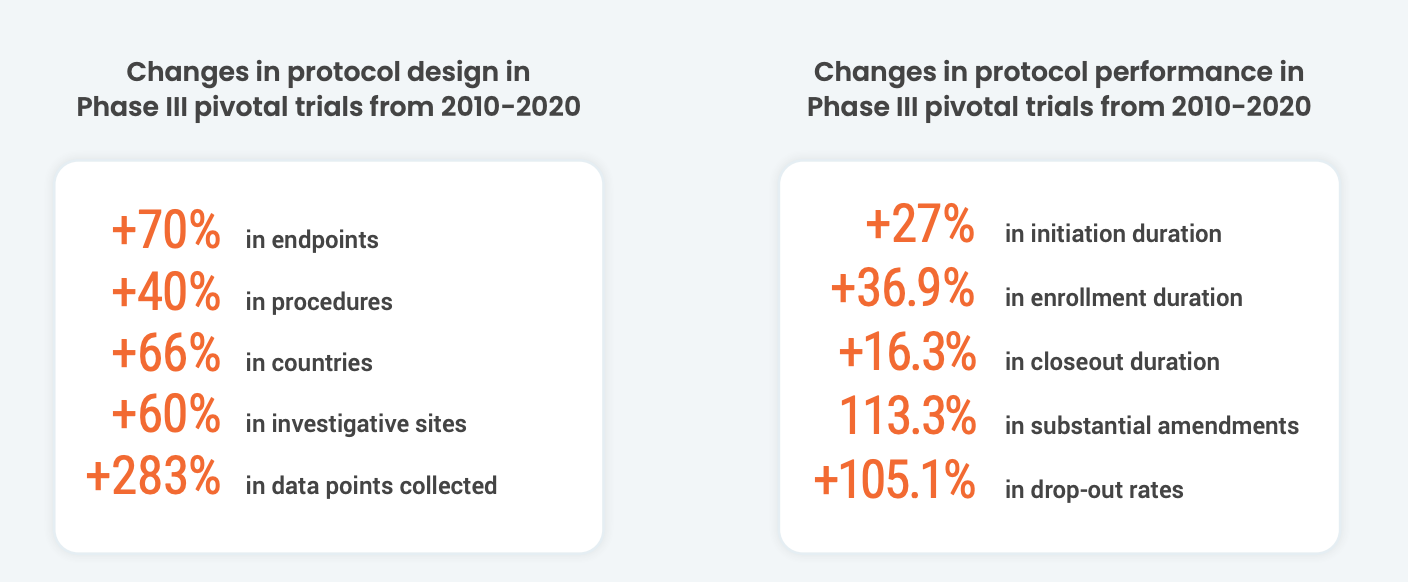
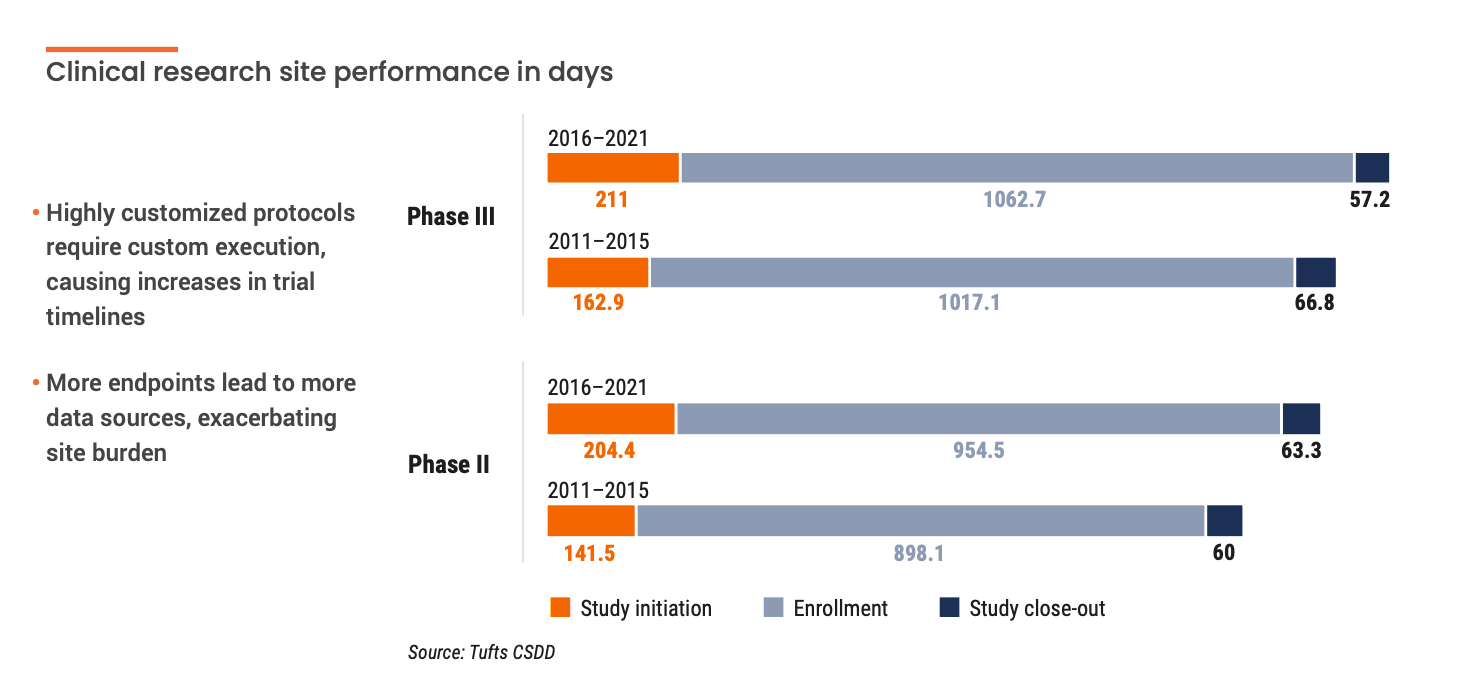
These complex protocols worsen existing technology silos for sites. According to a recent Society for Clinical Research Sites survey, over 60% of sites use more than 20 systems daily. On average, site staff spend 5-15 hours a month learning how to use new technology. This limits the time that site staff can spend with patients. This added stress can compound site burden. Grappling with chronic issues like difficult budgeting processes, low patient recruitment, and high CRA turnover results in lower-performing sites. This begs the question: how can companies help orchestrate and streamline technology for sites?
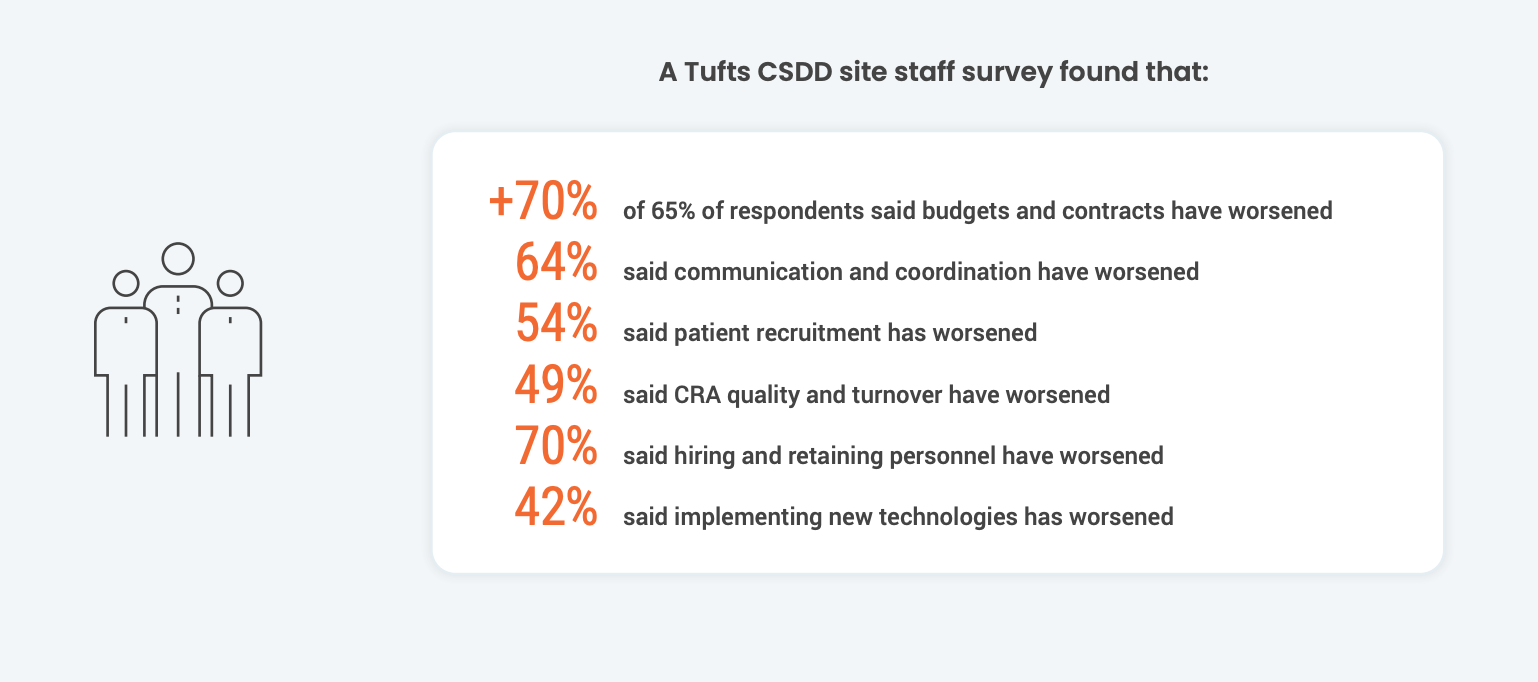
Building collaborative relationships with clinical research sites
As an industry pioneer in site-centricity, MSD has worked to address the perception that sponsors sit above their site partners and do not consider their needs. “To effectively partner with sites, we must give them a seat at the table and incorporate their valuable input into MSD’s decisions as a sponsor,” Natalie Blake explains.
MSD is moving away from process-driven site-sponsor relationships toward a consultative and dynamic mindset. The company implemented the Clinical Site Partnership (CSP) program to support this shift. The company has partnered with 30+ global sites to develop clinical trial technology strategies that work for site staff, sponsors, and patients. CSP includes focus groups, live site observations, surveys, workshops, and user experience sessions.
“As we design and develop new technology, we want to incorporate site experience to ensure what we are designing is meeting end-user needs.” - Natalie Blake, Director, Global Clinical Trials Organization, MSD
Since implementing this program, MSD has garnered feedback on over a dozen initiatives in areas like clinical supplies and data management. The company also implemented a “menu” of operational enhancements to make it easier for sites to conduct clinical trials. CSP sites deliver a high proportion of patient enrollment, including over 20% of MSD's oncology portfolio.
Sites participating in the initiative say that CSP opened lines of communication between MSD and site monitors. “As long as sites are willing and available, they will welcome the opportunity to collaborate with sponsors,” says one head of site management. Dr. Mustafa Erman, head of preventative oncology at the Hacattepe University Cancer Institute, echoed that sentiment. “Being a part of CSP has allowed me to communicate freely and frequently with MSD to optimize processes. It’s also improved screening, recruitment, and patient care.”
Clinical research sites in MSD’s partnership program enroll over 20% of the total oncology portfolio
Four steps to improve site engagement
Karen Whitson’s team at AbbVie has been focused on finding ways to improve their collaboration with sites, automate document exchange, and streamline their study startup process. In July 2023, her team launched a program-specific campaign using Veeva Site Connect with eight studies and 1,600 sites. Since then, they’ve seen:
- 100% of studies in the initial rollout now use Site Connect
- 410% increase in connected studies
- 78,488 safety letters distributed through Site Connect via email
Here are four steps Whitson’s team took that helped AbbVie improve site collaboration:
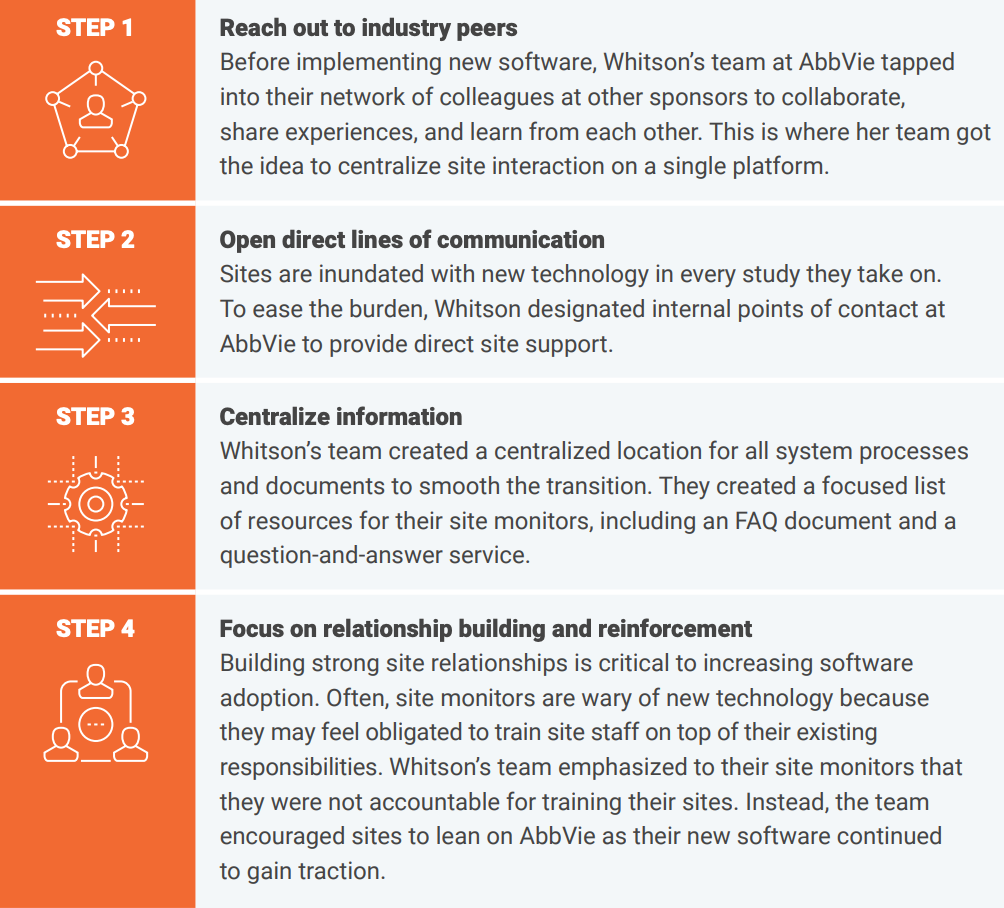
Sponsors who choose not to invest in technology can still prioritize site-centricity by establishing an advisory board or leveraging positive site relationships to gather input on trial decisions.
When searching for ways to improve site collaboration with technology, look toward unified solutions that automate information flow across trial partners, processes, and systems. Modern site collaboration solutions should have features like:
- Single sign-on to give sites easy access to all sponsor technologies through one ID
- A simple, free app to easily manage site content like ISF and delegation logs
- Centralized study logins to simplify access to sponsor technology
- Streamlined information and data exchange to improve collaboration between sponsors and CROs
Making a strategic effort to refocus on the fundamentals is the key to improving site collaboration in the long term. Engaging sites and giving them a seat at the table will lessen the transactional nature of site-sponsor relationships and streamline study execution. The site-centric approach can be straightforward: prioritize site input and foster collaboration to ensure better clinical trial outcomes for all.
Hear Jim Reilly, vice president of R&D strategy at Veeva, discuss ways companies can improve collaborations with research sites.