White Paper
Implementing Study Amendments Without Downtime or Data Migrations
Learn how to implement amendments in near real-time with Veeva Vault EDC.
The Challenge of Migrations in Clinical Trials
The growing complexity in clinical trials is driving an increase in protocol amendments. Research from the Tufts Center found that Phase III studies have an average of 3.6 protocol amendments, each containing 8.5 changes.1 That is over 30 individual changes in total. This count does not include other changes, such as corrections, updates to procedures, and new edit checks, meaning the total number of structural and functional changes is much higher.
For most studies, those amendments and other changes all required migrations. An article in Applied Clinical Trials describing how study complexity is directly correlated with the number of changes needed cited that studies with simple Case Report Forms (CRFs) required an average of eight migrations and those with highly complex CRFs saw an average of 17 migrations.2 These numbers are concerning when you consider the impact of changes and migrations on study timelines. According to one industry whitepaper, “The typical turn-around time for implementing a limited change, for example, adding 1 existing form in a new visit, is 10 working days.”3 Certainly the time required to implement the 8.5 changes in a typical amendment is much higher.
Why Changes Required Time-consuming Migrations
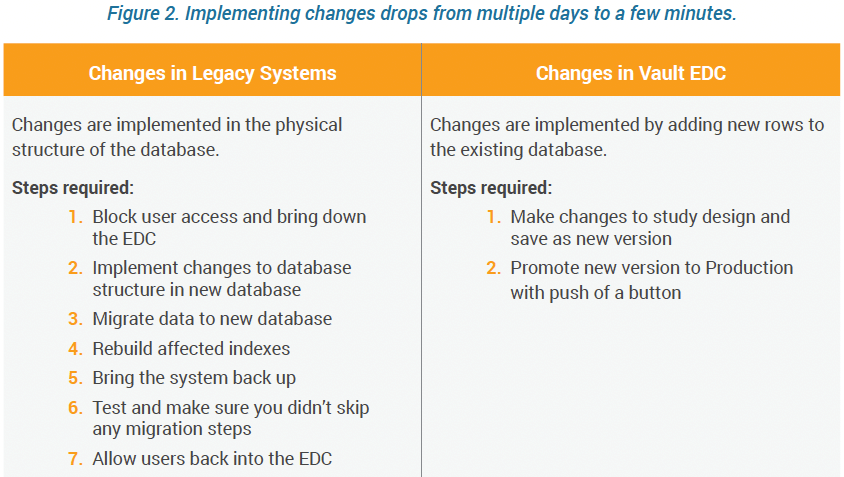
Implementing changes in traditional EDC systems takes a long time because organizations must build a new database and physically move existing clinical data. To achieve an effective data migration, data fields are mapped between systems, data is extracted from the old system and loaded to the new system.
After loading into the new system, results must be verified to determine whether data was accurately translated, is complete, and supports processes in the new system. During verification, you may need to run both systems in parallel to prevent accidental data loss while checking for disparities.
The system must be down or users blocked for the duration of all migration and test procedures. Thus, each migration introduces study downtime causing delays that can sometimes extend over several days.
The authors in the above mentioned ACT article advised companies to simplify their eCRFs in order to minimize the burden of migration costs and downtime. However, dumbing-down eCRFs creates risks around data quality, sub-optimal study designs, and poor alignment to increasingly complex protocols. This situation underlies the need for a better EDC, one that not only simplifies change management but embraces it.
A Modern Architecture and Approach in Veeva Vault EDC
A modern EDC allows for a casebook design that is as dynamic as the clinical trial itself. Veeva has delivered a next-generation EDC system, designed specifically to address the migration issue. Data is stored in a manner that provides flexibility and removes the need for migrations entirely. The ability to insert new data collection elements, new pages, and new visits, without bringing the system down or migrating data, simplifies the initial build as well as subsequent amendments.
A Flexible Architecture that Supports Change
With Veeva, all visits, forms, and fields are defined using a single data model with database tables and columns that do not change between studies. New studies and their eCRFs are defined by adding rows to the existing tables and columns. Once the study is live, you can add or change elements by adding new rows, without changing the database structure.
Vault EDC uses reference IDs as keys to the underlying data rather than using the physical location of the data. When clinical data is entered, it is stored with a reference pointer to the definition for that field. When a field is changed, a new reference is created for the stored value. As a result, the system can have multiple versions of a casebook at any time, there are no changes to the database structure, and study migrations are obviated.
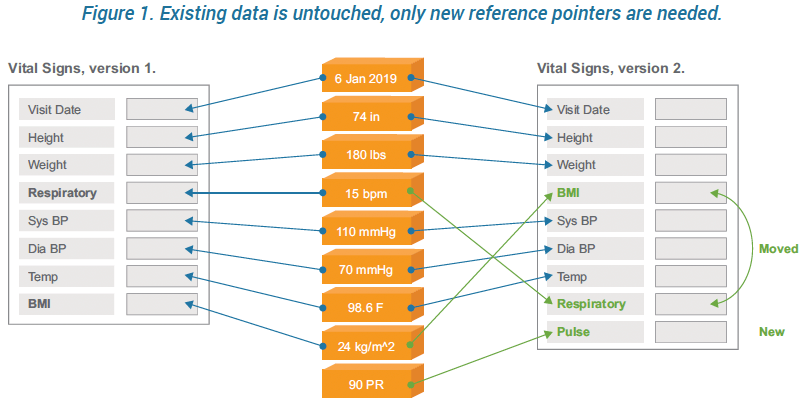
The Vault EDC architecture supports multiple CRF designs while adding/updating only the changes. When creating and deploying an amended casebook, any element of instantiated CRFs that are unchanged – remain completely untouched. Updates to instantiated CRFs only require a simple reference change to the new definition.
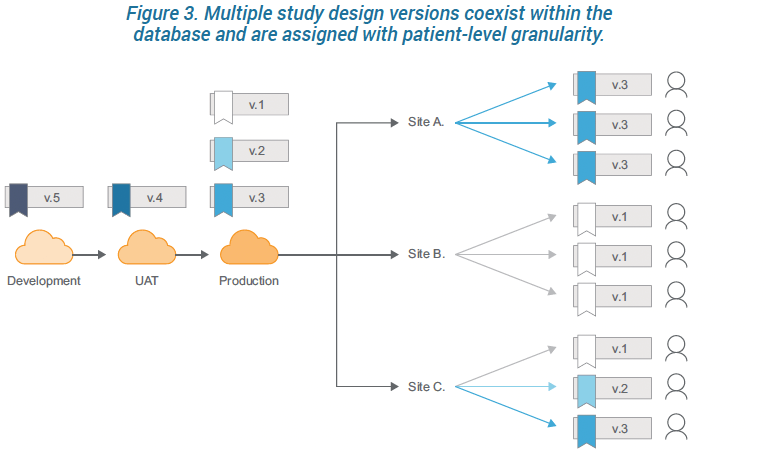
Prior versions retain their mappings, while new versions can introduce new mappings, thus every casebook can reference a different definition if need be. And different casebook versions can be active simultaneously. This flexibility extends all the way to the subject level. This design enables you to update individual sites and even individual subject casebooks with no site-level downtime.
Deploying Changes Without Disruption
Vault EDC saves you time and money by implementing changes in near real-time. When deploying a change, Vault automatically locks users out of a casebook for the few minutes it takes to effect the change and then releases the page. Normal service is resumed within minutes. And by scheduling deployments during nonworking hours at each site, there is zero disruption for sites.
The benefits from this flexibility are considerable: the desired changes go into effect faster, administration costs are minimal, and downtime for sites is eliminated. Perhaps most importantly, your trials become more agile and making amendments no longer poses risk to your data.
Learn how to increase agility in oncology trials with Veeva Vault CDMS.
2 Time for Another CRF Migration, Medidata Solutions, Applied Clinical Trials, December 2014. Accessed via: http://www.appliedclinicaltrialsonline.com/time-another-crf-migration
3 “Innovative approach to building an adaptive trial design in Medidata Rave®” Joris De Bondt, SGS Life Science Services, Paper TS07, PhUSE 2015.