EMD Serono Unifies Quality
Risk and Issue Management
EMD Serono is a business of Merck KGaA, Darmstadt, Germany
As a leading science and technology company, EMD Serono researches and develops drugs in several areas ranging from neurodegenerative and oncology, to inflammatory and autoimmune diseases.
EMD Serono’s R&D Quality (RDQ) organization wanted to implement a specific quality risk management approach to identify and address critical risks. The company adopted Veeva QMS to unify quality risk and issue management processes and meet their RDQ organization’s needs.
Unifying Risk and Issue Management
The company needed to transition from a reactive to proactive quality management, connecting quality and risk management to identify and mitigate critical risks up-front. This was not possible with their existing paper-based approach, and they needed a single, modern system to:
- Perform quality risk and issue management
- Support collaboration across quality and other functions
- Ensure compliance with data privacy standards
- Deliver consolidated real-time reporting
EMD Serono implemented Veeva QMS to streamline quality processes, and later, leveraged its Quality Risk Management (QRM) capabilities to incorporate risk management into their existing quality processes. A single system for issues and risk management enabled them to standardize risk identification, categorization, and mitigation across the organization, enhancing quality and patient safety across the entire product life cycle.
“We had two main challenges with the project,” said Nuno Galante Valério, Quality Strategy Lead – Digital Quality, RDQ at Merck KGaA, Darmstadt, Germany. “Fully integrating risk management into the overall quality system was a relatively new approach. And the team would have to merge two complex independent management processes in only eight months. But we knew we were breaking new ground and it would have high impact.”
Making this move allowed the company to make many improvements to their data model and quality management process. “This integration gave us a valuable opportunity to revisit our quality processes and rethink our approach to integration of core data elements with our quality data” Nuno said.
The RDQ team created a unified, risk-based approach with three main areas (Figure 1):
- Core quality activities such as audits and inspections
- “Issues events” such as observations, deviations, and changes
- The responses to quality issues like change actions, root cause analysis, and effectiveness checks
A risk management layer was added to integrate the second and third areas and all the data points in the system.
Figure 1: R&D Veeva QMS
EMD Serono QMS – Objects and Entities
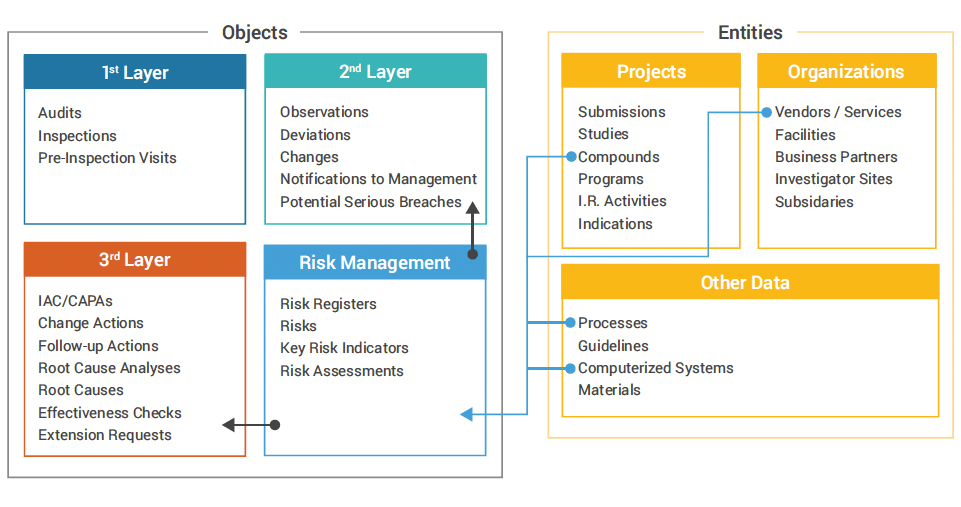
In deploying QRM, the RDQ team analyzed their quality processes to determine common elements between risk management and issue management. They then linked these interactions in the system to create a unified approach that could identify root causes and contributing factors on both sides.
“This approach enabled us to identify and reduce potential failures,” said Marion Pillwein, R&D Quality Risk Management lead for Merck KGaA, Darmstadt, Germany. “We are now analyzing risks, potential root causes, and categorizing them based on the root causes and reasons for failure,” Marion said.
Achieving True Data Integration and Transparency
EMD Serono now has a modern, streamlined risk-based quality management solution that consolidates real-time reports and ensures compliance with data privacy standards. Having a single source of truth enables the team to understand issues and their connection to risks, standardize risk scoring and tolerance, and proactively handle potential critical issues via mitigation actions for failure.
“A streamlined quality and risk management approach enables us to achieve true data integration and transparency on our risk appetite,” said Marion. “We achieved an increase on overall compliance by installing this important component (a unified approach) in our continuous improvement process. With risk control activities defined, we can take preventative actions to address potential quality issues and evaluate the effectiveness of our mitigations by monitoring the occurrence rate of these issues.”
More Customer Stories
Explore and learn more
Watch Video
Improve GxP Training Effectiveness with Veeva Vault Training

Read Customer Story
UCB Streamlines Change Control and Variation Management

Read Customer Story
Legend Biotech Increases Efficiency and Collaboration with Veeva Vault QualityDocs

