eBook
The Commercial Content Taxonomy for Life Sciences
Veeva creates a new industry standard
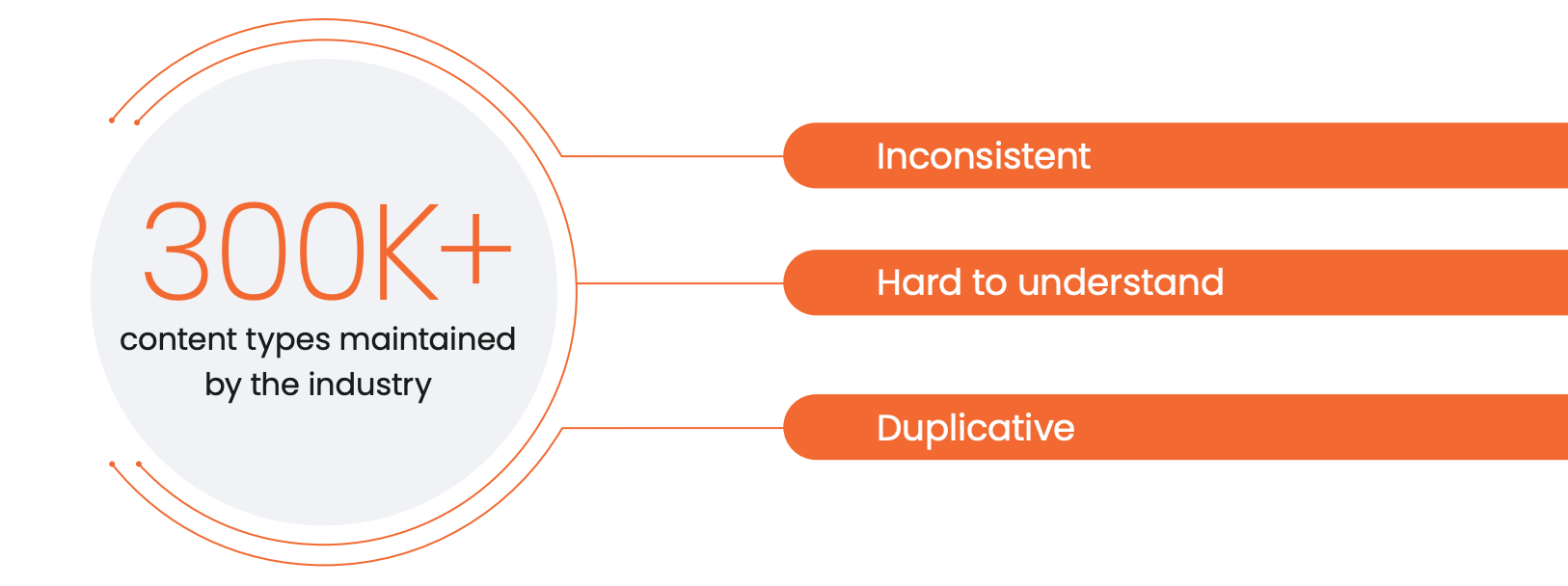
The life sciences industry currently maintains over 300,000 types of content. The historic absence of a standardized taxonomy means there is no common language to classify content globally.
Instead, existing content lists frequently mix up channels, intent, and format. Adding to the complexity, regulatory bodies tend to follow different approaches. Each authority classifies content distinctly: an internet banner in the U.S. might be considered a ‘banner ad’ in Canada, a ‘banner advertisement’ in the U.K., or ‘un bandeau internet’ (or ‘une bannière’) in France. Some authorities require a content type to be defined but many don’t. Others specify exact metadata or the type of document needed.
As a result, each life sciences company adheres to a customized naming convention. This inconsistency slows down our industry. For example, there are 68 ways to describe an electronic detail aid. Given that 60% of document subtypes are used only 10 times or less, end-users don’t fully understand how content has been categorized.1
Many of these subtypes were established before content tagging strategies and technologies became available. Administrators are trying to maintain these legacy data models, some of which are too high level, leading to millions of documents being classified in all-encompassing categories (such as ‘Promotional piece’, ‘MLR material’, or ‘Other’). Other models are too granular and result in thousands of unused labels. All of this impacts business productivity.
Did you know?
- 68 different ways to describe an electronic detail aid
- Legacy tags include ‘Fax templates’, ‘Bag stuffers’, and ‘Variable verbiage’
- 7,000 labels have never been used
- 60,000 subtypes used 10 times or less
- 14 acronyms beginning with ‘P’
To address these industry pain points, Veeva is creating a standardized taxonomy for — and with — life sciences. Our vision is that by 2030, the Commercial Content Taxonomy will be the established way of working and help the industry to be more efficient.
This global, industry-specific taxonomy will be published on our website so it is freely available to all. Our approach will make it as easy as possible for Veeva PromoMats customers to adopt the new taxonomy.
This white paper provides an overview of the taxonomy and explains how Veeva will enable industry adoption.
How will standardization help?
Most exciting innovations, including AI, are only feasible at scale when companies move from a customized system to a standardized approach. The Commercial Content Taxonomy will yield long-term benefits by:

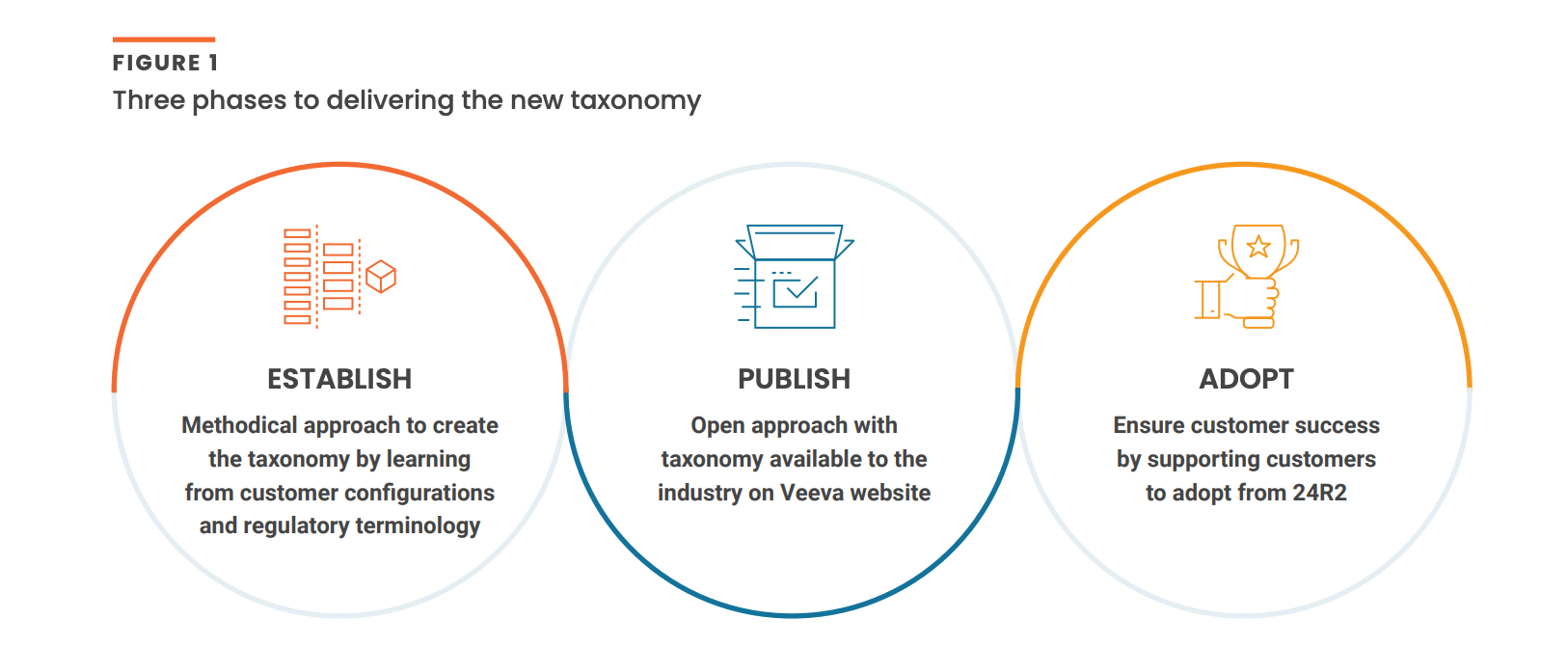
Three-phase approach: From concept to reality
There are three phases to developing and rolling out the Commercial Content Taxonomy [Figure 1]. First, Veeva will establish a comprehensive taxonomy by analyzing more than 475 customer configurations and reviewing regulatory terminology. Once the output has been developed and published, Veeva will work closely with customer advocates to raise awareness across the sector. From 24R2 onwards, new and existing Veeva PromoMats customers will be able to adopt the taxonomy within the product.
Phase 1: Establish
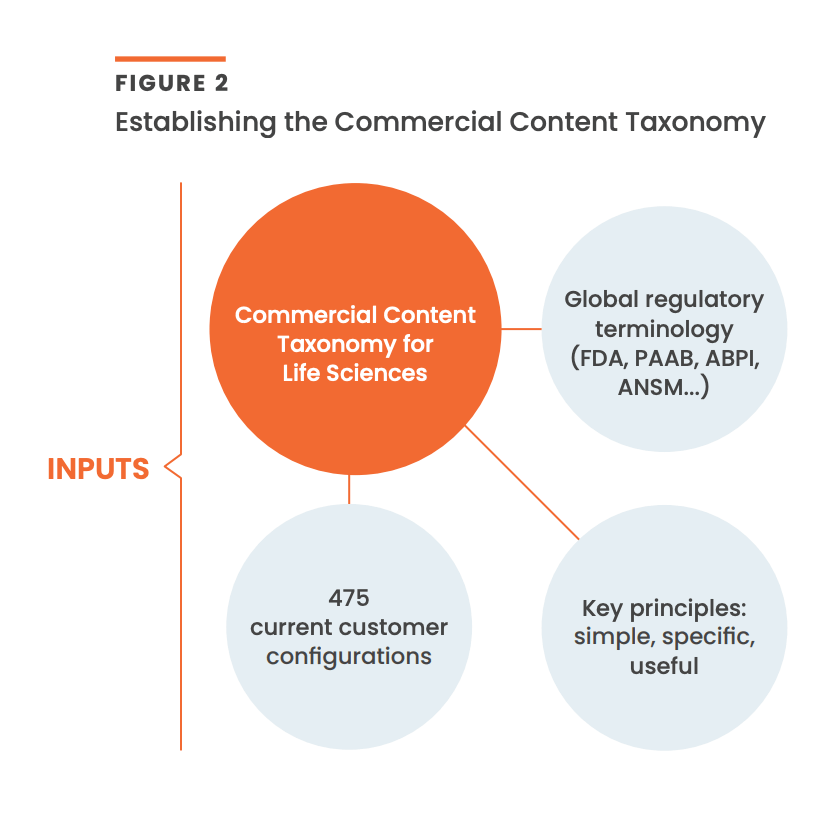
The guiding principles for the Commercial Content Taxonomy are that it should be simple, specific, and useful to life sciences. To create a taxonomy that delivers on these principles, Veeva will first analyze customer configurations in Veeva PromoMats spanning over 300,000 document types [Figure 2].
Given the majority of commercial content in life sciences is managed in Veeva PromoMats, this analysis will yield helpful conclusions for the industry. Global regulatory and industry body terminology (i.e., from the FDA, ABPI, ANSM, PAAB, etc.,) will inform the new standard, as will insights from discussions with industry experts.
The content hierarchy will extend to two levels: type and subtype. It will be based on content type (e.g., email, website) and have an accompanying description so there is clarity for users when classifying content. Companies will also be able to capture the intended use and audience in the document fields.
The taxonomy will span terminology used to classify documents (materials) and the building blocks used by life sciences companies to create these documents — including references used to substantiate claims, components key to digital asset management, and templates for creating content.
Phase 2: Publish
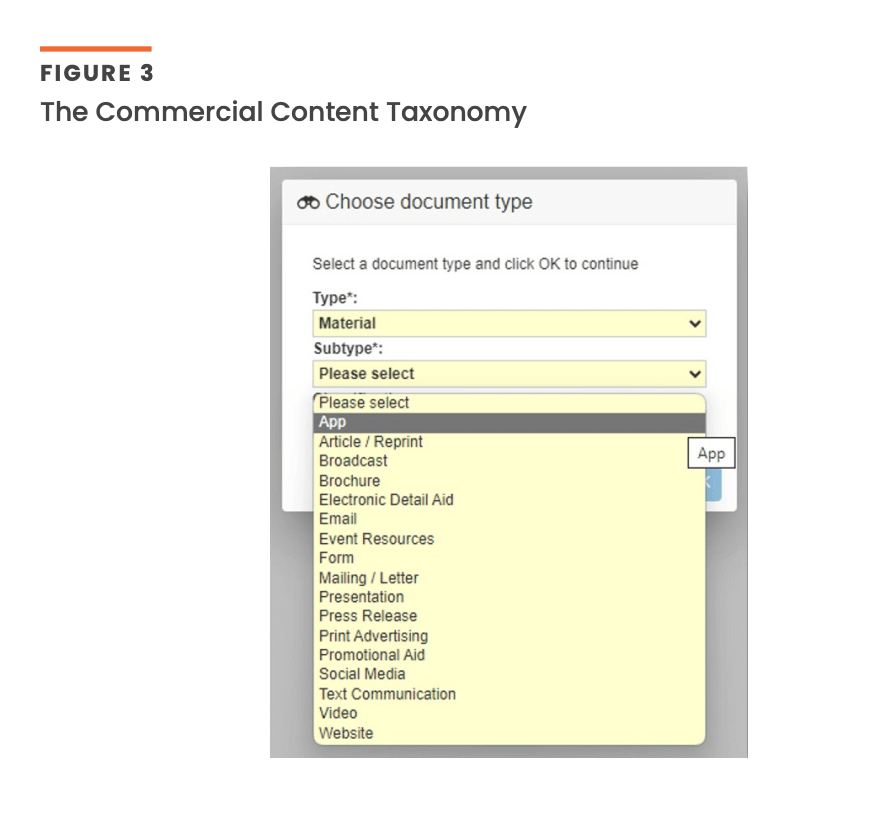
For simplicity, the Commercial Content Taxonomy will be a three-column Excel document covering Document Type, Document Subtype, and Description [Figure 3]. It will be published on the Veeva website so that the industry can download it and begin communicating in the same language immediately.
Who can access the Commercial Content Taxonomy?
The taxonomy will be available on Veeva.com to:
- Partners, agencies, and those working in life sciences content (including, but not limited to, Veeva partners)
- New and existing Veeva PromoMats customers, directly within the product (planned for 24R2)
Phase 3: Adopt
The Commercial Content Taxonomy will appear in Veeva PromoMats in 24R2 as a drop-down list of items when classifying a document.
For new customers, this drop-down will be set to ‘Active’ by default. Existing Veeva PromoMats customers will also be able to access it directly within the product but the drop-down will be set to ‘Inactive’ by default and end-users won’t see it. This way, customers can adopt the taxonomy at the right time for them.
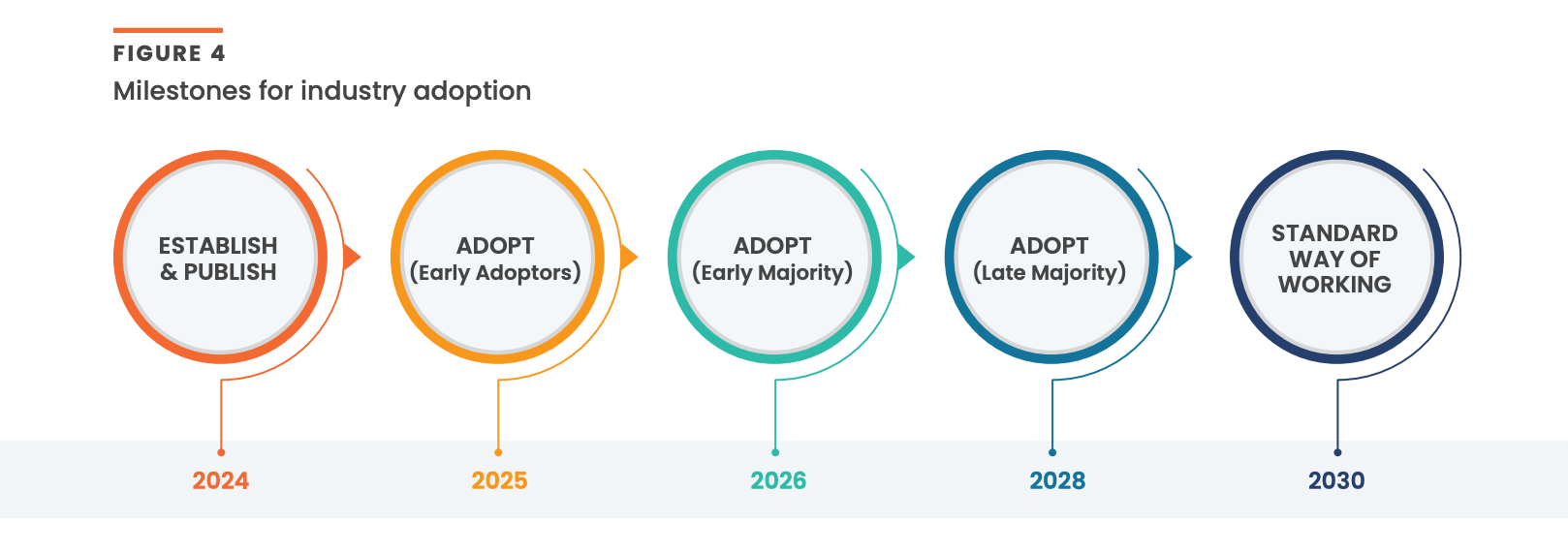
Our ambition is for most of the industry to use the model by 2030 [Figure 4]. To ensure we’re on the right track, we have launched a customer advisory group in North America and Europe, spanning from the largest biopharma to small and midsize companies. We also intend to educate global regulatory bodies on our proposed approach. New product features aligned to industry needs will be added incrementally as momentum grows.
How will Veeva support industry adoption?
Veeva will support the industry to adopt the taxonomy over time through:
- Education and enablement for partners (agencies, product, services, AI partners)
- Change program support (e.g., communication templates, analytics)
- Business process expertise to complement the technical aspects of the taxonomy
- Best practice documentation with specific recommendations
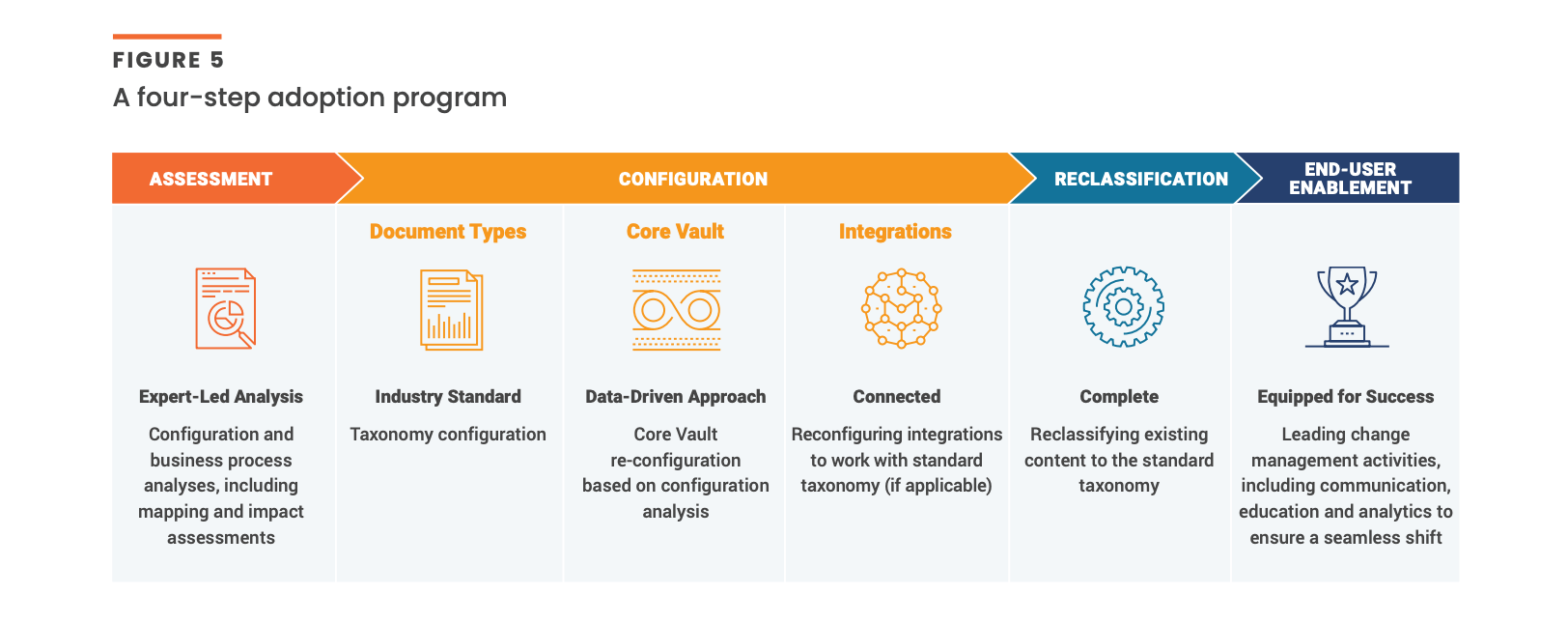
To help customers standardize their taxonomy, Veeva will offer a comprehensive four-step adoption program [Figure 5]. Our approach is designed to get customers up and running with the taxonomy quickly and easily while minimizing implementation resources.
During the assessment phase, our dedicated team will evaluate customers’ current taxonomy, establishing similarities and differences with the new standardized taxonomy.
Next, there will be a deep dive into the core Vault configuration with impact analyses completed in a wide range of areas (e.g., document types, metadata fields, lifecycles and workflows, etc.) Document types are core to the configuration, impacting workflows and integrations. The Veeva team deeply understands the considerations and implications and will make any required adjustments, configuring existing integrations to work with the new taxonomy.
During the reclassification phase, our team will provide customers with specific recommendations for reclassifying content to align with the standardized taxonomy before enabling end-users in Vault through training and change management support.
Next steps for using the taxonomy within your organization
A standardized content taxonomy will accelerate and simplify content management. It will also improve the user experience. Veeva’s 2030 vision is that the taxonomy helps life sciences to become more efficient and enables industry-specific content technology, such as AI.
The Commercial Content Taxonomy for Life Sciences will save your organization significant effort in defining the document hierarchy and taxonomy. To facilitate this process, we will help customers build the business case, while our services team will run a dedicated four-step adoption program including specific recommendations to align reclassified content with the new taxonomy.
Veeva is the global leader in the cloud software life sciences industry. Committed to innovation, product excellence, and customer success, Veeva serves more than 1,100 customers, ranging from the world's largest biopharma companies to emerging biotechs. As a Public Benefit Corporation, Veeva is committed to balancing the interests of all stakeholders, including customers, employees, shareholders, and the industries it serves.
For more information, visit www.veeva.com.
1 Based on data from Veeva PromoMats