White Paper
White Paper: Streamline Change Control for Generics Organizations
Drive continuous quality improvement by connecting people, processes, and technology
Today’s Reality: The Broken Change Control Process
Approved product changes impacting multiple products and business functions require all parties to coordinate to record and exchange information on time. However, because of separate processes and systems, business functions communicate via phone calls and emails, making cross-functional collaboration difficult. Miscommunication among stakeholders often leads to change execution without full impact assessment, resulting in downstream activities that are not justified by the benefit of the change.
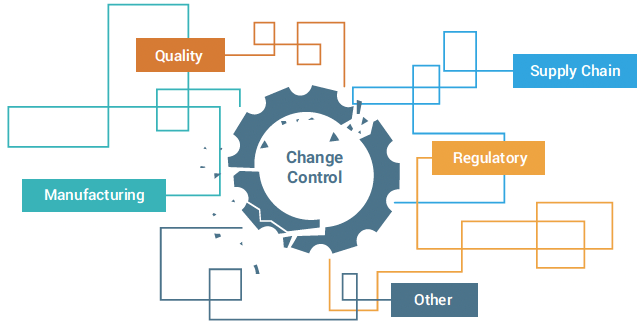
This broken and inefficient change control process increases compliance risk and delays product ship decisions.
Unifying disconnected systems and processes on a modern cloud platform builds the foundation for change control transformation. This paper provides a holistic approach to streamlining the change control process to improve quality, compliance, and time-to-market.
The Opportunity: Connecting People, Processes, and Technology
Streamlining change control requires improvements in three core areas: people, processes, and technology. Optimizing the relationship between these three elements increases efficiency and accelerates product shipments.
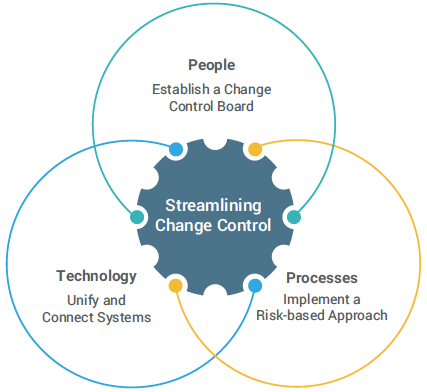
People: Establish a Change Control Board
Driving a successful transformation project requires buy-in from key stakeholders across all business functions.
A change control board can help establish the right governance model and gain cross-functional alignment. This central function can drive a global and consistent change control process, bringing clarity and improving collaboration. A streamlined change control process that supports all types of changes can also help prioritize, bundle, and implement changes most cost-effectively.
Processes: Implement a Risk-based Approach
Not all changes are equally important; bringing all stakeholders across all changes reduces agility and lowers productivity.
A risk-based approach simplifies and speeds the change control process while maintaining the right level of quality oversight.
Start by classifying risks in different tiers depending on the nature and extent of the changes. Each change category specifies the level of involvement for various stakeholders. For example, while a facility change may involve regulatory, supply chain, manufacturing, and IT stakeholders, a minor system update may involve only IT and quality teams.
Technology: Unify and Connect Systems
Change control teams need the right intelligence at the right time at each step of the change control process, and modern technology can enable that.
Unifying multiple processes and applications on a modern cloud platform lets you manage and track the entire change management process in a single workflow. Three unification opportunities to automate and accelerate the change control process are:
- Unifying and connecting quality systems
- Connecting change control and variation management
- Enabling supplier collaboration and visibility
Unifying and Connecting Quality Systems
Disparate document management, change control, and training systems create various disconnect points across the change control process. A quality system landscape with complex cross-application integrations cannot enable full automation, increasing manual tasks to manage the change control workflow and duplicate data entries.
A unified quality environment automates and streamlines the change control process by eliminating manual tasks and the need for complex integrations.
UNIFIED CHANGE MANAGEMENT
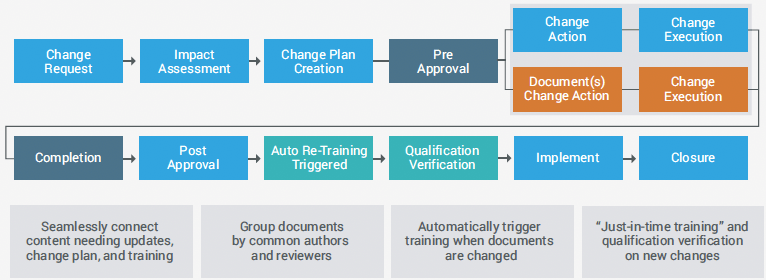
For example, substituting equipment in the manufacturing process may require SOP changes. Having change control and document management in one system can automatically identify impacted documents and trigger a document change action.
Once documents are updated and approved, the system can trigger a training action to re-train users on the new procedure.
This automation, multiplied across hundreds of changes per year, brings significant efficiencies. More changes can be resolved faster, reducing the time spent following the “paper trail” and ensuring a thorough documentation update process and timely user training.
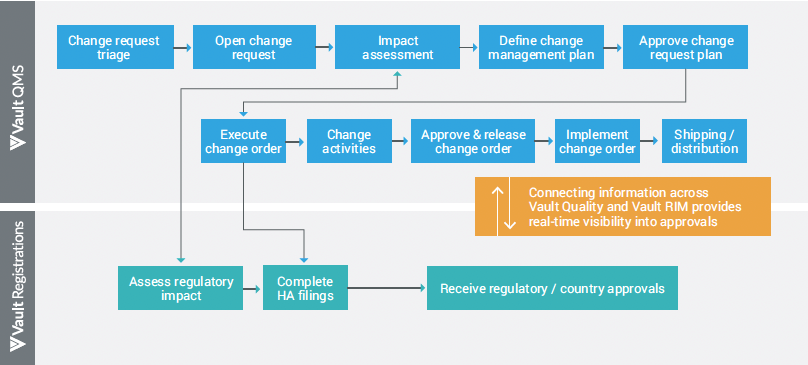
Connecting Change Control and Variation Management
Managing regulatory impact assessment through a separate variation management process causes lack of transparency and critical errors across change control activities.
Connecting the change control and variation management processes through a single automated workflow eliminates duplicate data entries, speeds data exchange, and ensures regulatory impact assessment before the change approval action.
For example, a seamless connection between Veeva Vault QMS and Veeva Vault Registrations applications ensures that a change to a product or system is introduced in a controlled and coordinated manner. It provides quality teams with the full visibility into health authority filings and approvals, expediting the product release process.
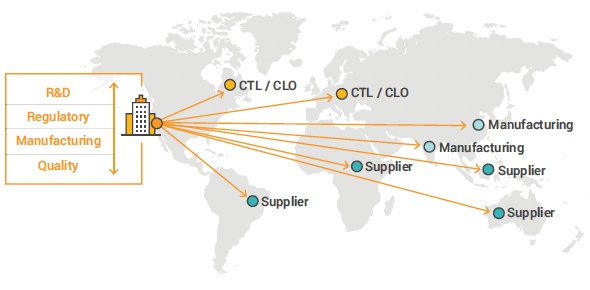
Enabling Supplier Collaboration and Visibility
As the reliance on external suppliers and partners continues to increase, integrating them into quality processes is essential to optimize manufacturing cost and bring drugs to regional patients faster.
A unified cloud platform like Veeva Vault can bring the complex ecosystem of suppliers into the change control process, improving collaboration and providing end-to-end visibility. User-level permissions and built-in data integrity controls enable you to provide secure access to suppliers, making it easier to get the required information for change approval and monitor their change implementation activities.
INCORPORATING INTERNAL AND EXTERNAL PARTIES
Bidirectional Communication with all Critical Stakeholders
The Result: Streamlined Change Control
The holistic approach to streamlining the change control process enables you to:
- Increase efficiency: The right intelligence at the right time allows you to assess the scope, priority, and timing of changes. For example, you can bundle multiple related changes together to increase resource efficiency and optimize cost.
- Reduce cycle times: Real-time access to data and intelligence across regions and markets minimizes product distribution delays and helps you avoid a short supply situation. Trends and visibility into previous changes provide valuable insights to improve the change control process and reduce cycle times further.
- Ensure regulatory compliance: A complete understanding of the regulatory impact before change execution allows companies to make informed decisions about implementing changes. The transparency into the health authority approval process enables quality teams to plan when and where to ship products, minimizing errors and regulatory risks.
Conclusion
Streamlining change control requires optimizing people, processes, and technology. It starts with aligning the right stakeholders across business functions and establishing a central change control governance model. A cross-functional group also helps with implementing a risk-based approach to change control.
Unifying and connecting change control processes on a common cloud platform minimizes manual intervention and reduces cycle times. With Veeva Vault Quality Suite, companies can prioritize and manage inventory effectively, improve manufacturing throughput, and bring products to patients faster.
To learn more, visit veeva.com/quality.