Site Perspectives:
5 Key Insights to Become
a Sponsor of Choice
Clinical research sites weigh in on how sponsors can improve working relationships
Clinical research sites are the backbone of drug development, but their numbers have dwindled amid the increase in protocol complexity and proliferation of fragmented technology solutions. This forces sites to adopt dozens of sponsor-specific tools and build customized workflows around them, increasing site burden with manual and error-prone processes.
These compounding challenges have reached an inflection point: 55% of sites report that their top challenge when participating in trials is supporting a variety of technologies. Sites need consistency to better serve patients, and companies who answer the call will position themselves as sponsors of choice in a competitive market.
In this report, representatives from clinical research sites – including Lurie Children’s Hospital, ClinOhio Research Services, Skylight Health, and more – discuss how sponsors can improve site relationships.

#1 Give sites standard ways to work across sponsors
A recent SCRS survey found that only 5% of sites say sponsor technology meets their needs “very well.” A significant contributor to this challenge is the proliferation of one-off point solutions, leaving sites with countless applications, logins, passwords, and portals. On average, sites use around 12 distinct technological components in each trial.

Theresa Oswald, director of research operations and conduct at Lurie Children’s Hospital, says that if sponsors can reevaluate their systems landscape to see where they can consolidate, it would lessen that burden. “Technology can ease some of our struggles by streamlining our workflows, like helping us keep track of what’s due without needing to sort through emails,” she explains.
For example, many sponsors have individual solutions to manage study documents, safety letter distribution, payment letters, and end-of-study media. Instead, sponsors can look for solutions that unify these capabilities under one login. This way, sites can have everything they need in one place to help them stay informed and close out tasks in just a few clicks.
“If a coordinator or a site administrator can log in and see the things that are due each day without having to search for them in email or try to remember who sent them, that would save time,” says Oswald.
Although these process improvements may seem small, they can have a big impact on site staff and patients. Alisha Garibaldi, CEO of Skylight Health Research, has seen the impact firsthand. “The patient-facing aspects of our job are very important, but there’s so much we have to do on the backend to get in front of our patients,” she explains. “ Taking away a couple of hours per week that we spend working on regulatory matters will give us more time to connect with patients, improve our follow-up rates, and make sure that our trial results are valid.”

#2 Approach sites when ready
Tina Bowdish, senior director of clinical research at a cancer research center, recommends that sponsors are truly ready before approaching sites to launch a study. “It’s great to get site feedback before you launch a study. But, when you’re trying to initiate a trial and the sponsor doesn’t have all of their vendors and documents completed, it makes it really difficult on sites. It lengthens our start-up process,” she says.
Sponsors can administer industry-standard feasibility questions to create site and principal investigator (PI) profiles, which reduces the number of times site staff have to answer the same questions, improves the start-up experience, and helps identify the right sites for a study faster.
Time saved in study start-up has a positive impact on patients. “I am at a pediatric site and we do a lot of rare disease trials,” says Oswald. “The time it takes for study start-up is really impactful to our families. They are waiting for these studies to enroll their children so they can get a lifesaving treatment.”
Bowdish adds: “If sponsors could make sure that they are truly ready before they approach us about a study, I think we could significantly cut down on long study start-up timelines.”

#3 Develop clear guidance for sites beyond the protocol
Sponsors can proactively share better guidance to equip sites for their trials. This can come in many forms, including designated sponsor points of contact, centralized locations for system processes and documents, and guidelines to supplement the protocol.
One example from Brad Hightower, founder of Hightower Clinical, is in the development of electronic case report form (eCRF) guidelines.
“Sometimes the protocol is not clear enough; it asks for vitals but may not tell you that you need three sets of vitals, five minutes apart,” Hightower says. “Sponsors should provide a combination of the protocol and any supporting guidelines needed — and we shouldn’t have to ask for them.”

#4 Prioritize meaningful study training
When it comes to study training, a more intentional approach makes things easier for site staff. For example, a common scenario for site initiation training involves gathering site staff in a room to review a PowerPoint presentation with screenshots of each section of the protocol.
Instead, sponsors can consider replacing that time-intensive experience with an interactive package of online materials, including a video overview of the compound and a quiz on the protocol. The latter is more effective and efficient, enabling the sponsor to assign the training when it is relevant to the trial milestone. It also gives site staff the freedom to complete training at a time that works best for them.
Then, sites and sponsors can focus on more meaningful activities during initiation and other face-to-face interactions, such as:
- Answering PI questions on protocol or dosing
- Reviewing enrollment criteria
- Relationship building
“Site initiation should be a short series of meetings versus one big slide presentation,” says Hightower. “All of it is helpful, but some of it would be far more helpful beforehand or could be resolved before a four-hour slide presentation.”

#5 Streamline document exchange
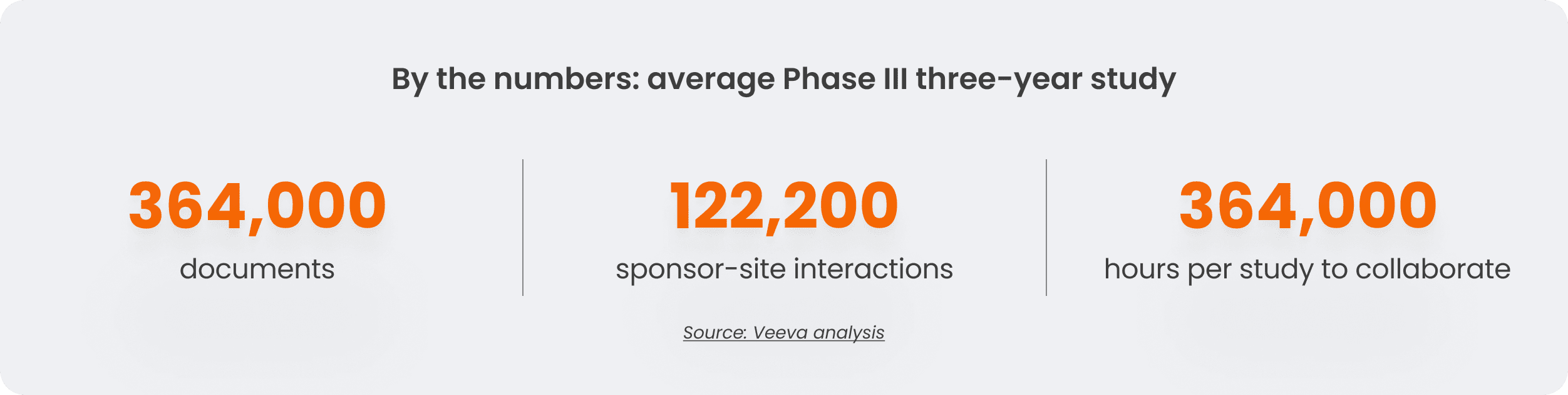
One of the most taxing parts of site-sponsor collaboration is document exchange. For example, a single three-year study with 200 sites can accrue an estimated 364,500 documents and 122,200 transactions, such as emails and chats. This amounts to 10,000 hours spent sharing information per study.
Jim Sanders, president and founder of ClinOhio Research Services, struggled with the manual effort it took to send information back and forth. “One of the biggest challenges during study start-up is making sure that all the documents that the sponsor requests are completed properly and sent back to them promptly,” he says. “When you’re looking at these massive zip files of regulatory documents from the sponsor via email, that can get challenging. Sometimes you don’t even have the space to store them on your computer. Or, the emails bounce back because the files are too big.”
With a dedicated site-sponsor collaboration solution, ClinOhio has cut down on its email usage during study start-up. In one study, the team went from sending 140-150 emails to the sponsor down to only 30-35 — a roughly 75% decrease in daily email volume. ClinOhio also shortened its response time from 6-7 days to just 2-3. “We’ve had a better relationship with the sponsor because of this. We’re not getting frustrated with them and they’re not pinging us for the same documents because it’s all right there.”

Hightower adds: “I would love to see us get out of email communication and into a platform where things can be tracked. We receive multiple emails with zip files for regulatory matters and send a spreadsheet back and forth for budget negotiations, both of which introduce errors and inefficiencies.”
Improving site engagement
Sponsors should look for unified solutions that automate information flow across trial partners, processes, and systems to improve site collaboration. Modern site collaboration solutions should have features like:
- Single sign-on to give sites easy access to all sponsor technologies through one ID
- A simple, free app to easily manage site content like ISF and delegation logs
- Streamlined information and data exchange to improve collaboration between sponsors and CROs
- High-value training where sponsors assign tasks automatically before site visits and track on-site activities
Making a strategic effort to engage sites and give them a seat at the table will streamline study execution and elevate companies to a sponsor of choice. An effective site-centric approach can be straightforward: prioritize site input and foster collaboration to ensure better clinical trial outcomes for all.

