Veeva OpenData Compliance Data
Streamline Compliance with
Healthcare Professionals
Engage with confidence. Report with accuracy.
Learn how Alnylam Reached a Broader HCP Audience
Veeva OpenData Compliance Data
Veeva OpenData provides real-time NPI and compliance data to keep your team compliant with rules for pharma rep detailing, sample eligibility, and spend transparency.
Benefits
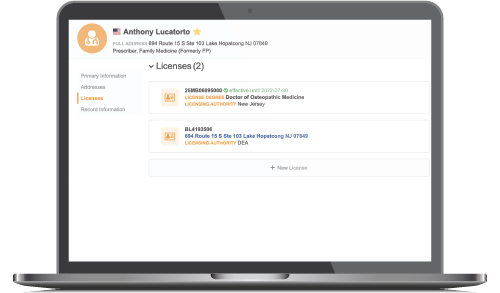
Enforce Sampling Protocols
Access verified healthcare provider NPI and sample eligibility data for compliance with state and local regulations.
Minimize Off-label Promotion Risks
Verify NPI and specialty information to detail products and adhere to Corporate Integrity Agreements.
Report with Accuracy
Gain visibility into cross-border activity and reconcile aggregate spend.
Features
Robust Compliance Data
Keep pace with rapidly changing compliance requirements at the state and local level.
- Get verified NPI and specialty data for compliance with PDMA, DEA, OIG, LEIE and Ohio TDDD legislation
- Use a single, global ID to track activity with healthcare professionals no matter where you engage
- More effectively enforce sampling protocols
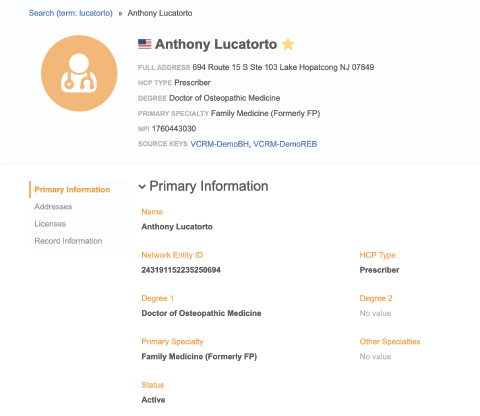
Immediate Specialty Verification
Minimize the risk of off-label promotions and adhere to Corporate Integrity Agreements.
- See specialty designations and credentials including MD, DO, NP, and PA degrees
- Confirm sample eligibility before the call
- Minimize the cost of wasted samples
Spend Transparency
Trace cross-border engagement for compliance reporting.
- Reference a unique global ID for all reportable entities
- See accurate roll-up reports across geographies
- Reconcile aggregate spend
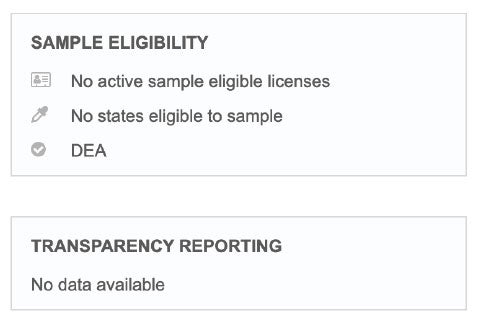
Continuous Quality Updates
Rest assured in the accuracy of data with proactive data quality services.
- Receive timely, ongoing updates from Veeva data stewards
- Submit data change requests (DCRs) directly from Veeva CRM
- Get your DCRs fact-checked and processed within one business day








