Veeva QMS
Standardize Quality Processes
with Veeva QMS
Achieve better control and visibility into quality processes
with accelerated validation and built-in best practices.
Announced 2016
Status MATURE
Customers 100+
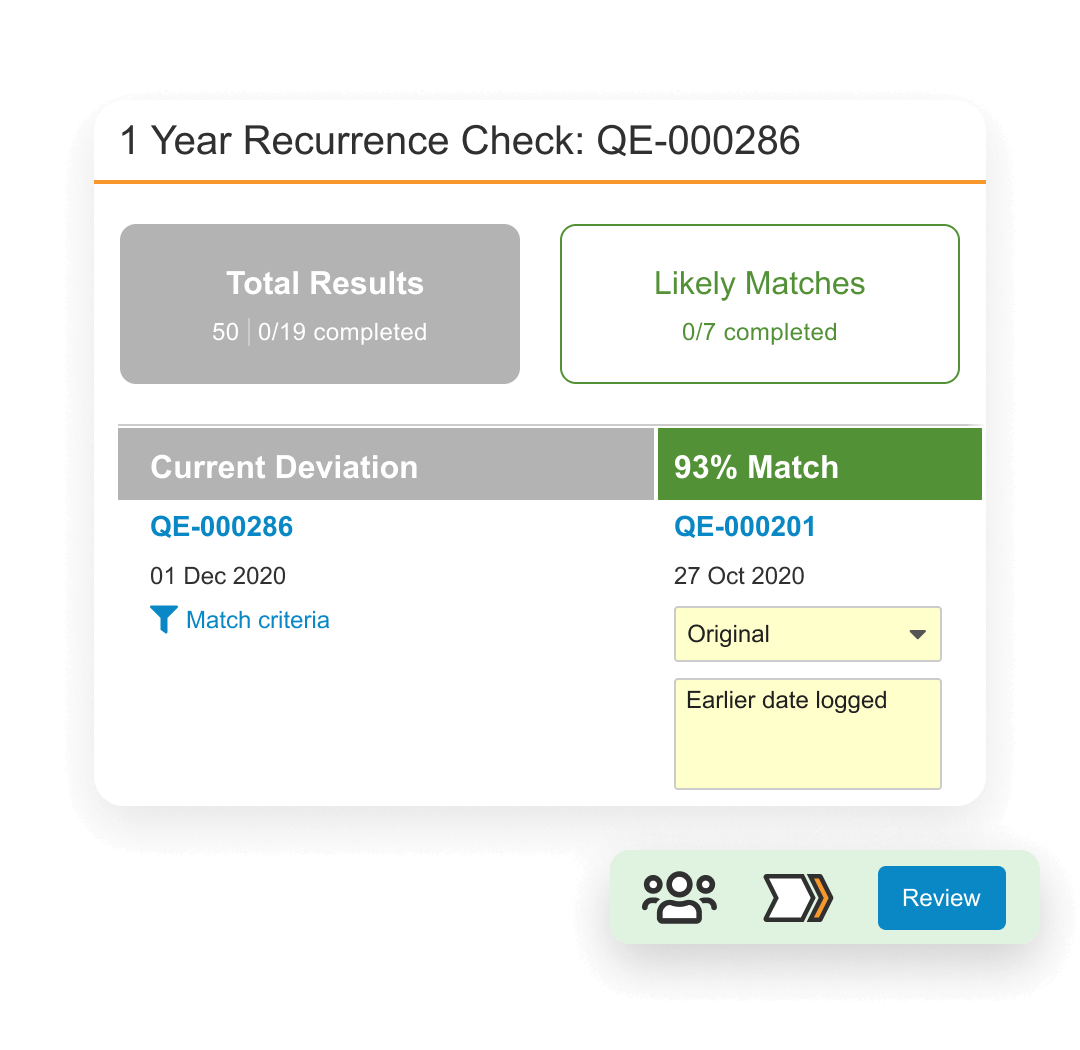
Veeva QMS is a system designed to manage life sciences-specific quality processes. It provides best practices for handling quality processes such as deviations, audits, and continuous improvement. It also allows external partners to access the system to collaborate on audit findings and supplier corrective actions.
Veeva QMS is unified with other Veeva Quality applications and connected to Registrations to enable coordination of product change control activities. Additionally, QMS and Veeva LIMS bring together quality assurance and quality control processes in one system.
Why Veeva QMS
Standardize quality processes globally
Global alignment and control
Easily bring internal and external teams and partners into continuous improvement processes.Rapid time-to-value
Increase operational efficiency with automated workflows and built-in best practices.Enable real-time risk-based decisions
Improve risk visibility across products and processes with a unified approach to risk management.Unified quality management
Improve speed, efficiency, and GxP compliance with a unified suite of quality applications.







